Thermochromic materials: pigments, inks, paints
Materials that change color with temperature
OliKrom is the leader in custom thermochromic products that change color with temperature.
Every day, we design and produce custom pigments, liquid crystals and/or dyes, and formulate inks and paints with guaranteed thermochromic performance.
On request, we adjust transition temperatures, reversibility/irreversibility, color selection...
15 years of expertise in the field of thermochromic coatings
Our custom thermochromic products
In the world of thermochromism, we have made the strategic choice of "custom". Examples of commercial thermochromic liquid crystals, dyes and coatings are numerous, as are the mechanisms behind thermochromism. However, each generation has its advantages and limitations.
As soon as you send us a request (activated transition temperature, colors, type of resin or solvent), our teams study your specifications, your industrial environment, your regulatory and economic constraints etc.
This analysis allow us to select the generation compounds best suited to your request and your deposition technique (screen printing, spray...).
We then produce your customized thermochromic pigment (liquid crystal and/or dye), then we formulate the ink and/or paint.
The heat-reactivity of the coating is controlled at each step, and rectified if necessary, to deliver at the end of the development a material that meets your expectations.
Our pigments and custom thermochromic dyes
With 15 years of experience in the world of thermochromism, our expert chemists and engineers master all the thermochromic families: organic, hybrid and inorganic.
We have a large variety of thermochromic compounds in powder form, and our internal research leads us to continuously enrich this alphabet by exploiting the mechanisms of structural modification of isomerization, cyclization (or ring opening), transfer of electrons, protons, hydrogen atoms and/or spin states...
By experiment, we distinguish three main classes of behavior, depending on whether the thermochromism is reversible, with memory effect, or irreversible:
Thermochromism
reversible
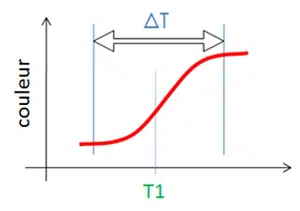
The thermochromic property is reversible and progressive, centered around the activated T1 value. The transition temperature T1 can be adjusted from -100°C to +1000°C.
Thermochromism with
memory effect
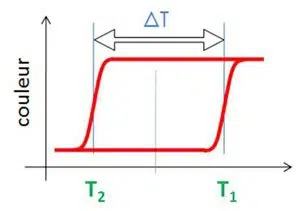
The transition occurs at T1 temperature and returns to its original color when the temperature drops to T2. The difference between T1 and T2 defines the memory effect of the thermochromic property.
Thermochromism
irreversible
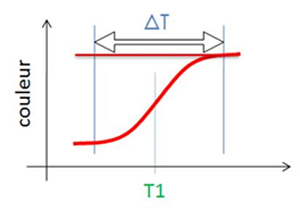
The irreversible transition is definitive as soon as the temperature rises above a defined level T1. The irreversible threshold value T1 can be adjusted from +60°C to +900°C.
By molecular engineering, our team program the material (powder pigment & dye) as closely as possible to your project. To obtain the reversible character, the memory effect or the irreversibility, we finely modulate the proportion of organic entities (such as "liquid crystals" or "leuco dyes") or inorganic entities within the pigment & dye:
- The organic material provides the necessary degree of flexibility to ensure the speed of the phenomenon and the absence of fatigue for the colors change.
- The inorganic component provides the "light resistance".
It is a subtle balance on pigment and dye to create, control and adapt the thermochromic property to your need, your targeted product. For example, a change from colorless to red by increasing the temperature.
Every day, our teams create new custom thermochromic pigments and dyes. Some developments lead us to adjust the transition temperatures of materials from 0-100°C, others concern higher temperatures of 300-1000°C, or on the contrary pigments that are reactive at low temperatures: close to nitrogen or even helium.
Other developments at the scale of the powder pigment (dye) lead us to adjust the speed or the fatigability of the thermal transition, the activated colors: colorless, red, yellow, green, colorless, black...
Our custom heat-sensitive inks and paints
In the field of thermochromic powder pigment, the formulation stage (preparation of inks and paints) is critical. It is too often neglected due to a lack of knowledge of the physical processes involved: material science, phase transition, thermodynamics...
We know that the application of shear forces associated with the passage of the tricylinder and/or the ball mill can alter the crystallinity of pigment particles (as well as crystals, leuco-dyes...). That a simple additive of a resin (of solvent), that a simple variation of the polarity of the medium and/or the pH changes the thermodynamic equilibrium at the origin of the phenomenon of color change, or the optical properties by modifying the respective positioning of the electronic states.
This ultra-reactivity of thermochromic pigment (liquid crystal, leuco-dye...) to their environment, obliges us to check at each stage of the formulation (from powder to resin transformation), the maintenance of the smart property.
This also explains why :
- we create our own heat-sensitive pigments (liquid crystal, leuco-dye...),
- but only supply our products in the form of inks, varnishes and paints,
- to guarantee the performance of the coating, the application of the thermo-sensitive product (spray gun, airbrush, screen printing...).
All our materials are manufactured in our ISO 9001 certified industrial site in Pessac, France, according to rigorous manufacturing processes and under the permanent control of our quality manager.
Each manufacturing batch is traced and accompanied by a performance certificate. Any deviation is recorded, analyzed and corrected as part of our continuous improvement policy.

Thermochromic ink
Nature: solvent-based or UV photocurable ink
Support: to be defined according to the project
Application: Screen printing - Flexography - Pad printing - Offset

Thermochromic paint
Nature: solvent or aqueous phase
Support: to be defined according to the project
Application: Spray - Brush - Roller - Aerosol can
By experience, we know the main pitfalls encountered with thermochromism: the choice of the transition temperature, the loss of properties during formulation (transformation of the pigment, of the liquid crystal and/or leuco-dye, from the powder to the solution, to the formulated paint) and/or during the industrial process, the sensitivity to light, the low tinting strength, the fatigability of the coloration transition during thermal cycles, the limited thermal resistance of the pigment (200-240°C), the toxicity of some components, etc.
In the world of thermochromism, our team accompanies you from A to Z, from the idea to the final product. This allows us to intervene effectively at each stage of the project: research and development, pre-industrialization and industrialization.

Industrial safety and predictive maintenance
In the industrial safety and predictive maintenance (energy, petrochemical, nuclear...), the use of a thermochromic coating offers multiple possibilities of application. The coloration change of an ink (resin, paint) allows dynamic temperature control without any wiring or electricity supply as opposed to a thermocouple whose fragility is prohibitive for certain uses.
As example of application, a heat reactive indicator/coating (changing from colorless to blue or from yellow to black...) can be used to monitor the surface of a motor to warn of an overheating condition, an electrical element to detect a short circuit, a tank to visualize its filling level, an industrial pipe, valves, ovens... to control the occurrence of an anomaly.

Aeronautics, space & defense
The use of thermochromic materials allows, for example, to detect an anomaly, to control the health of composite structures and the risk of delamination inherent to an abnormal rise in temperature.
In the Defense, the issue can be the camouflage of a structure, capable of adapting to its environment, like a chameleon. For instance, a camouflage structure, capable of altering its appearance, may be defined by a color-changing layer.
Originally, the structure has a first color state and after the color changing layer changes the color by thermochromic effect, the camouflage structure has a second color state. For example, a transparent or semi-transparent color changing layer capable of reflecting or emitting red light could be arranged on the woody camouflage layer, which changes the overall appearance from greenish woody camouflage to brownish desert camouflage. On request, we can adjust the balance of yellow, brown, green, black...

Medical and food field
The use of thermochromic technology makes it possible to control the thermal evolution of an element, to reinforce the traceability as to detect a rupture of the food cold chain, to attest the conformity of storage of a drug, a vaccine...
The presence of an thermochromic resin capable of changing color with temperature (i.e. in blue, orange, yellow, violet, green, dark...) is understandable by all. It is a simple way to track down an anomaly easily in the storage of food, drug, vaccine.

Smart packaging, anti-counterfeiting and authentication
Is your bottle of wine at the good temperature for tasting? The label in contact with the bottle will tell you, by a change of color (for example from colorless to black or green...) using an ink (screen printing...) and/or a thermochromic paint (spray...).
It exists a large range of thermochromic examples : other indicators of well-being, of good use, exist on beer bottles, take-away containers, cups...
Thermochromic coatings open up a new Eldorado for the creation of smart packaging. The use of smart packaging allows to create an interaction with the consumer, to boost the image of a brand...
The thermo-reactive products, and more generally smart coatings, offers the possibility to increase the complexity of the packaging and to create a wide range of security keys of different levels depending on the desired protection, to reveal a counterfeit, to authenticate products...
![]()
CONTACT US
We will get back to you as soon as possible..
"*" indicates required fields

