All about photochromic materials
What is a photochromic material?
A photochromic products (from the Greek photôs light and chromos color) is, by definition, made up of pigments/dyes whose optical properties tune during illumination: like eyeglass lenses that switch from transparent to dark under the effect of sunlight.
These photosensitive products are part of the vast family of X-chromic derivatives, i.e. pigments and dyes that change colors under the effect of an external excitation : temperature, light, pressure, humidity level, etc.
What is the mechanism of photochromism ?
 More specifically, a photosensitive product is a substance, organic (mainly with aromatic units), hybrid, polymer or inorganic, whose coloration is modified under the effect of an excitation following the conversion of ground state into a metastable excited level. This photochromism phenomenon can be reversible or not, and the kinetics is strongly dependent on the compound, the nature of the wavelength and the light intensity.
More specifically, a photosensitive product is a substance, organic (mainly with aromatic units), hybrid, polymer or inorganic, whose coloration is modified under the effect of an excitation following the conversion of ground state into a metastable excited level. This photochromism phenomenon can be reversible or not, and the kinetics is strongly dependent on the compound, the nature of the wavelength and the light intensity.
Photochromism at the molecular level is a reversible transformation, photo-induced in at least one direction, between two states A and B of a species with different absorption spectra and a quantum yield of efficiency. The electromagnetic radiation inducing the transformation (evaluated with a quantum yield of efficiency) must belong to the UV, visible or near IR range.
The A → B transition is accomplished by excitation at a wavelength λA corresponding to an absorption spectra of the isomer in the A ground level, and that of B → A by irradiation at a wavelength λB in the absorption spectra of the molecular isomer in the B photostationary (metastable or stable) excited level. In some cases, the B → A transition can be obtained by thermal reaction (Δ).
Depending of the return reactions from B to A, there are two types of molecular isomers: - type P, if the return takes place only optically; - type T, if the return can also take place thermally.
What are the main families of photochromics materials?
Historically, the first description of photo-induced reactions dates back to 1867. Fritzsche (C. R. l'Academie Sci. 1867, 69, 1035) reported the decoloration of a tetracene molecule in sunlight, then its return to its initial color (orange) in the dark.
Today, photochromism is a property observed in many materials, like biological systems notably associated with the mechanism of retinal vision. Photoisomerization events in vision involve the protein opsin molecule and the cis/trans isomers of retinal. The cis-retinal fits into a receptor site of opsin. Upon absorption of a photon in the visible range, cis-retinal can isomerize to all-trans-retinal.
Reactions that do not require large conformational changes during the photochemical reactions can be observed in solution as well as in crystalline phase.
Organics photochromic molecules
They are certainly the most studied family of photochromics. The processes involved during illumination, which lead to colors changing, are numerous. It may be a structural transition (photoisomerization around double bonds, proton transfer, opening and closing of rings), as a redox process.
Photochromism by redox reaction was discovered in the quinone and naphthalene family (Z. F Phys. Chem., 1899, 30, 140). In these systems, the optical transition is caused by the redox reaction which strongly changes the conjugation of the arenes.
Cis-trans photoisomerization is encountered in the aromatic derivatives of stilbenes, azobenzenes. They present an photoisomerization of the double bond C=C or N=N under UV irradiation. An example of photoisomerization is the "Disperse Red one" (DR1).
Intramolecular proton transfer isomerization is described for salicylidene-aniline systems. Intramolecular proton transfer allows the transition from the enol unit (yellow) to the ketone form (colored in red due to an n-p* transition of the oxygen free electron pair). Depending on the compound, the return to the enol unit may take a few seconds or several months.
Ring closure or ring opening isomerization is a mechanism encountered in two isomers:
- T-type group (thermal reversibility) such as spiropyrans and spiro-oxazines
- P-type group (photochemical reversibility) such as fulgides and diarylethenes
Spiropyrans and spiro-oxazines have been widely studied. Under ultraviolet, the carbon-oxygen bond breaks, followed by a cis-trans isomerization leading to the colored form called merocyanine.
The first example of reversible diarylethene was described by Irie (J. Org. Chem. 1988, 53, 803-808). This family is now widely studied. The diarylethene molecule combines both thermal irreversibility and high strength. Photocoloring and photodecoloring cycles can be performed on some diarylethenes more than 104 times without observing photodegradation (J. Org. Chem. 1988, 53, 6136-6138).
Hybrid photochromic structures
Ligands with photosensitive antenna
Many hybrid molecules are composed of one (or more) organic photochromics antenna(s) linked to a metal ion. The conformational change of these antennas (dyes) by photoisomerization and/or photo-induced cyclization reactions can cause the coloring modification of the molecule.
Among the most described photochromic antennas are azobenzene, spiropyran, diarylethene or quinone derivatives. The ligands used to connect the metal complex are frequently bipyridine, terpyridine as well as cyclopentadienyl substituted by an azobenzene group.
If we consider as an illustration the diarylethenes associated with complexes, the obtained compounds present the photochromic property thanks to the photocyclisation mechanism of the ligand. The described photochromics compounds involve rhenium, platinum and zinc.
Geometry changing photochromic structures
The most prominent example is the copper complex, [Cu(dieten)2](BF4)2 having two N,N-diethylethylenediamine (dieten) ligands. Under excitation, the complex changes from a square planar conformation to a slightly distorted conformation that places the coordination site in a slightly tetrahedral conformation. The color change from red to purple is stable for several hours at 35 K.
The photosensitive products with spin transition
Some derivatives have the particularity to modify their spin state under the effect of a photochemical process. The transition from a high spin to a low electron spin configuration may be accompanied by optical modification. There are several types of transitions caused by light excitation. The LIESST (Light-Induced Excited Spin State Trapping) effect is the most studied photoinduced effect. It was first observed in 1984 (Inorg. Chem. 1985, 24, 2174) in the iron(II) complex with six propyltetrazole (ptz) ligands, [Fe(ptz)6](BF4)2.
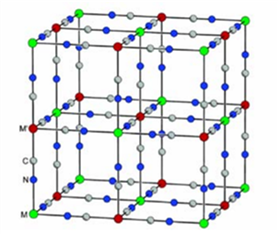 Mixed valence photoreactive systems
Mixed valence photoreactive systems
Some Prussian Blue analogues are known for their photochromic and magnetic properties. These complexes, composed of two divalent and trivalent coordination centers connected by cyano ligands, show a magnetization induced by light excitation. This is notably the case for cobalt-iron analogues (Polyhedron 2001, 20, 1339) which switch from the FeIIILS-CN-CoIIILS to the FeIILS-CN-CoIIHS form by excitation at low temperature. This transfer of electrons from one coordination center to another is accompanied by a structural transition, with a modification of the absorption spectrum from green to red and a modulation of the magnetic properties.
Photoreactive molecules with intraconversion of a ligand
The most studied compounds of this family are the complexes with a nitrosyl ligand, such as sodium nitroprusside : Na2[Fe(CN)5(NO)].
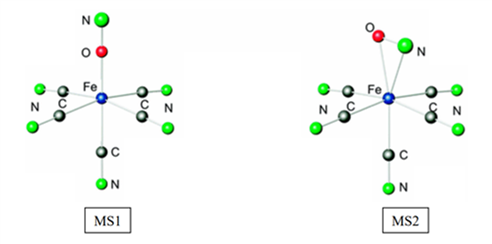 This nitroprusside complex has two metastable states:
This nitroprusside complex has two metastable states:
- an MS1 level which is characterized by the inversion of the nitrosyl into an isonitrosyl ligand, where the metal is directly coordinated to the oxygen.
- an MS2 level, for which the linear nitrosyl ligand in the ground state becomes "bent". In this so-called bent conformation, the iron-ligand bond is considered a η2 -NO conformation.
Inorganic photochromic products
The phochemical has also been described in inorganic systems, notably, n silver halides, zinc and molybdenum oxides.
Under the effect of light excitation, the redox reaction, Ag+ + X-→ Ag0 + X0 (X = Cl, Br, I) leads to the formation of silver colloids. The initially colored material becomes dark gray. When the excitation ceases, the reverse reaction takes place. The material returns to its stable configuration and recovers its initial coloring.
Another class of inorganic derivatives is that of molybdenum-based oxides. Under UV excitation (of sunligh for instance), MoO3 changes from pale yellow to an intense blue. The modification is due to the photoreduction of metal cations Mo6+ (4d0 configuration) to Mo5+ (4d1 configuration) under illumination.
Conclusion
Through this brief analysis of the existing, we have just reported the main pigments and dyes, and mechanisms responsible for the photochromism. Each of these families has advantages and disadvantages.
The photo-induced effect is dependent on the chemical process involved, and four main factors must be taken into account:
- the intensity and nature of optical source: wavelength, sunlight, continuous or discontinuous, laser...
- the conversion rate from the ground state to the photostationary state (evaluated with a quantum yield of efficiency),
- the lifetime of the photostationary state,
- the temperature range,
- the nature of the environment: powder, crystal, polymer, solution, ink, paint,
- the fatigue of the process.
These main factors vary according to the nature of the chemical process and the nature of the molecules.
Our chemists and photochemists at OliKrom (research department, process department of smart inks and paints, production unit and advice and assistance) are at your disposal to select the best molecule according to your specifications.
We create our own pigments, dyes and deliver our coatings in the form of inks, varnishes and paints to guarantee the performance of the smart product on its support.
![]()
CONTACT US
We will get back to you as soon as possible..
"*" indicates required fields

